Peptide Drug Conjugates (PDC)
With their small molecular size, superior tissue penetration, and efficient synthesis process, Peptide Drug Conjugate (PDC) are emerging as the next major advancement in tumor-targeted therapy following ADC. The global PDC market is projected to exceed $12.843 billion by 2030 at a 19.2% CAGR*.
At ChemExpress, we leverage our extensive peptide services and cutting-edge payload-linker technology platform to provide end-to-end solutions for PDC drug development. Partner with us to accelerate your PDC pipeline from R&D to commercialization!
Source: Grand view Research: Peptide Drug Conjugates Market Size, Industry Report, 2030

One-Stop PDC
CDMO Service
ChemExpress offers integrated services spanning from Payload-Linker, peptide synthesis and conjugate to PDC DS&DP , enabling efficient scale-up and seamless technology transfer.

Extensive Project Experience
& Comprehensive Inventory
ChemExpress has over 80 payloads, over 400 linkers, over 1,000 linker syntheses experience and over 1,500 peptide projects completed, assisting in the synthesis of over 300 PDC compounds

Regulatory Compliance
ChemExpress offers ISO9001-aligned R&D and GMP-compliant production, meeting FDA/EMA/NMPA regulatory standards, ensuring high-quality, scalable solutions for your PDC pipeline.
Our Services
ChemExpress provides a comprehensive range of services from R&D to commercialization, supporting your PDC projects at every stage. Contact with us today for faster, more reliable, and safer PDC drug development.
Discovery Synthesis
Peptide High-Throughput Synthesis
- 36/48 Multi-Channel Peptide Synthesizer, Simultaneously Synthesizes of 48 Peptides of Different Sequences
- Scale: 0.05-0.1 mmol (1-10 mg) Per Channel
- Sequence Length: 3-60 AA
- Automated Purification Collection
- Rapid Delivery : 2 weeks/cycle (20-30 mer)
Peptide Customized Services
- Solid-phase Synthesis and Liquid-phase Synthesis
- N-terminal Modification: Acetyl, Carbamate, PEGylation, Biotinylation, Fluorescent Labeling, etc
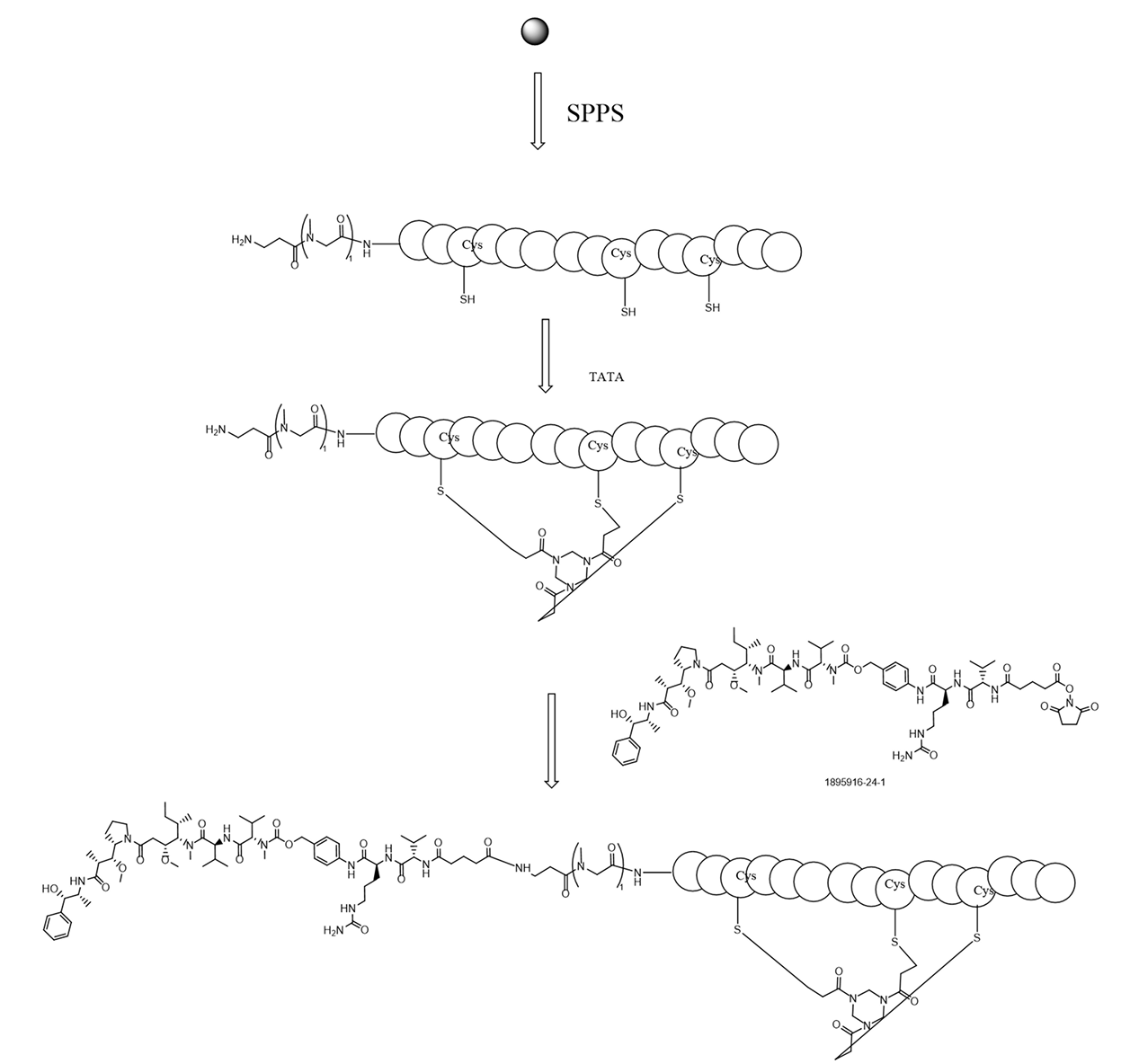
- Cyclic Peptides Synthesis: TATA Cyclization, 1 to 2 Disulfide Bonds, 2 to 3 Disulfide Bonds, Click Cyclization, Amide Rings, Click and Disulfide Double Rings, Stapled Peptides, etc
- C-terminal Modifications Camides, Esters, AMC
- GLP-1 Derivatives
Peptide Quality Control
- Impurities Studies
- Chiral Impurities Analysis
- MS/MS-sequencing, N-sequencing, NMR, Amino Acid Composition, Corresponding Isomers, Assay, Solvent Residues, Heavy Metals, Microorganisms, Endotoxins, etc.
Payload Linker Synthesis & Analysis
- Microtubulin Inhibitors: MMAE, MMAD, MMAF, DM1, DM4
- Targeting DNA Payloads: PBD
- Enzyme Inhibitors: Dxd, SN38, Exatecan
- Cleavable Linkers / Non-Cleavable Linkers
- Process Parameter Optimization
- Stability Studies on Unstable Intermediates
- Purification Method Research
- Genotoxic Impurity Studies
- Process Characterization & Validation
Development & Manufacturing
PDC Drug Substance Development
- Conjugation Process Development and Validation
- Scale-Up Process Development
PDC Analysis
- Structure Characterization
- Quality Release
- Stability Studies
- IND/ NDA Filling
PDC Drug Product Development & Manufacturing
- Pre-formulation Studies
- Pre-stability Studies
- Formulation Screening
- Compatibility Studies of Excipients and Packaging Material
- Freeze-drying Process Development and Optimization
- 2ml, 20ml, 50ml Aqueous Injections and Lyophilized
Why Partner with ChemExpress
Cost Effective
Synthesis Capability
Our Sites
R&D Site & Manufacturing Site
Case Study
In an active-ester PDC conjugation route, conventional conditions led to insufficient cyclization and coupling, limiting purity. By integrating solid-phase synthesis with optimized reaction parameters, we achieved a cyclization yield of 91% and a coupling conversion rate above 85%, enabling consistent gram-scale PDC production with >98% purity.
FAQs
Our peptide synthesis platform integrates solid-phase and liquid-phase synthesis, with high-throughput and automated production lines for rapid screening and synthesis. We can synthesize peptides ranging from 3 to 80 amino acids and offer synthesis scales from milligrams to kilograms. We also support the development of cyclic peptides (including multiple cycles), fluorescent labeling, and other functionalized peptides.
We produce peptides at scales ranging from gram to kilogram per batch, with both Non-GMP and GMP production lines. Our GMP production facilities feature explosion-proof synthesis areas, and purification and lyophilization processes are conducted in Class D cleanrooms. The discharge area can be upgraded to Class D-A, enabling API-grade peptide production.
Chiral impurities refer to non-target stereoisomers formed during peptide synthesis due to racemization or other causes. These impurities can impact the peptide's efficacy, safety, and stability. Particularly in clinical applications, chiral isomers may lead to reduced drug activity or potential side effects.
The control of chiral impurities is achieved through the following strategies:
· Starting Material Control: Ensuring high purity of starting materials and setting strict quality standards to prevent introducing impurities from substandard materials.
· Process Control: Screening optimal reaction conditions (such as temperature, solvent, pH) to minimize racemization and reduce unwanted chiral impurities.
· Process Strategy:Implementing specific process strategies, such as using chiral reagents or enzyme-catalyzed reactions, to reduce racemization risk and ensure high chiral purity in the final product.
Structural characterization of peptides is essential to ensure product quality and functionality. We employ a range of analytical techniques, including nuclear magnetic resonance (¹H-NMR), MS/MS-sequencing, N-sequencing, two-dimensional NMR (2D-NMR), infrared spectroscopy (IR), and ultraviolet spectroscopy (UV). These complementary approaches verify the structure, sequence, and purity of peptides, ensuring accuracy and reliability.




