ADC CDMO
-
Antibody–drug conjugates (ADCs) are a promising cancer treatment modality that enables the selective delivery of highly cytotoxic payloads to tumors. However, realizing the full potential of this platform necessitates innovative molecular designs to tackle several clinical challenges such as drug resistance, tumor heterogeneity and treatment-related adverse effects.
Several emerging drug conjugates formats—such as Peptide–drug Conjugates (PDCs), Radionuclide-drug Conjugates (RDCs), Degrader-Antibody Conjugates (DACs), Bispecific ADCs, and dual-payload ADCs—offer unique capabilities for tackling these challenges.
Choosing a professional ADC CDMO partner ensures that these complex modalities can be translated from concept to clinic efficiently.

One-Stop ADC
CDMO Service
ChemExpress offers integrated services spanning from Payload-Linker to ADC DS&DP, enabling efficient scale-up and seamless technology transfer, effectively reducing management and transition costs.

Extensive Project Experience
& Comprehensive Inventory
Over 160 ADC projects have been delivered, including over 60 CMC projects, 5 BLA projects, and 1 commercial project. 15 ADC payloads and related intermediates are registered with the FDA as DMFs.

Expert Scientific
Team
Our team of over 500 scientists provides comprehensive support from R&D to commercialization, ensuring high-quality, scalable solutions for your ADC pipeline.
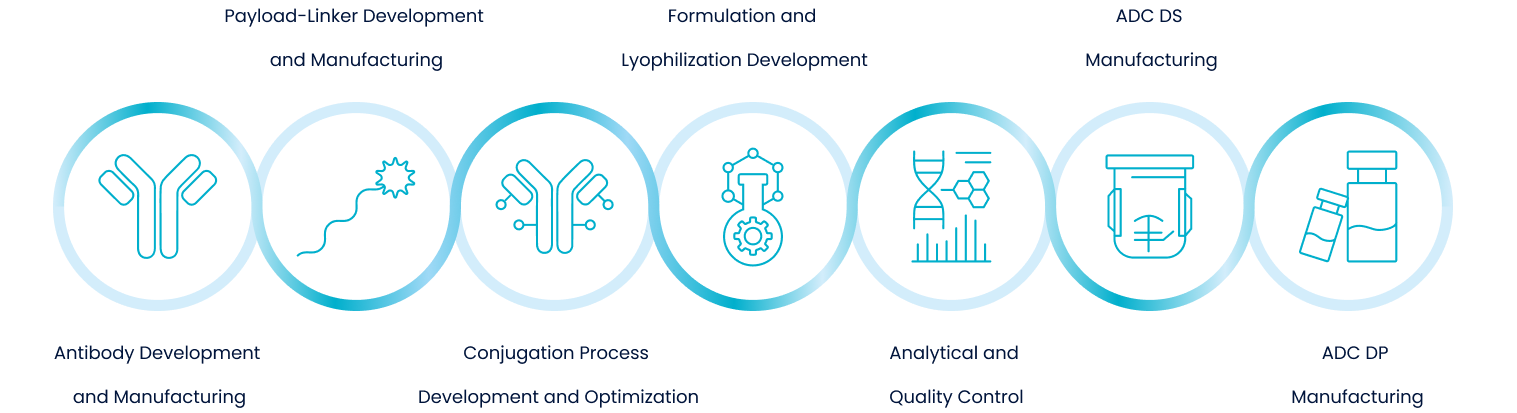
Our Services
Conjugates(ADCs,PDCs,RDCs,DACs)
At ChemExpress, we offer comprehensive and customizable conjugation services to support the development of a broad range of conjugated drug modalities (XDCs), including ADCs, PDCs, and other emerging bioconjugates.
Our services are tailored to meet the unique needs of pharmaceutical and biotech partners at every stage—from early discovery and process optimization to clinical and commercial manufacturing.
- Site-specific & Non-site-specific Bioconjugation Technologies: Cysteine-based conjugations (-SH), Lysine-based conjugations (-NH2), N-Glycan Conjugation and Enzyme conjugations.
- Service Type: Fee for Service (FFS), Full-Time Equivalent(FTE), and Chemistry, Manufacturing, and Controls (CMC)support.
- Conjugate Drug Screening
- Conjugation Process Development and Optimization
- Conjugate Sample Preparation
Payload-Linker
ChemExpress leads the field for Payload-Linker. Our proven expertise, extensive inventory, and robust GMP infrastructure enable us to support XDC projects from R&D to commercialization.
- 80+ Payloads in Stock, 400+ Linkers in Stock, 1,000+ Linker Syntheses Experience
- 60+ CMC Projects,5 BLA Projects, 1 Commercial Project
- 5 GMP HPAPI production lines (OEB5, OEL Down to 10ng/m³)
- Total Volume over 4,000 L, Scale up to Kilograms (Commercial Scale)
- 14 ADC payloads and related intermediates are registered with the FDA as DMFs: MMAE, Vc-MMAE, Exatecan, Eribulin, and etc.
Biologics
ChemExpress provides comprehensive, full lifecycle solutions encompassing monoclonal, bispecific, and multispecific antibodies. Leveraging the ADC CDMO expertise, antibody DS manufacturing can be seamlessly integrated with conjugation workflows, enabling efficient ADC development and manufacturing.
Antibody DS GMP Manufacturing
- 1 production line, ~2,900 L capacity
- Produced in 200 L, 500 L, 2,000 L sizes
- Annual design capacity: 120,000L
ADC DS GMP Manufacturing
- OEB-5 isolator, adapted to a wide range of ADC conjugation processes
- 50 L, 100 L, 200 L, 500 L Disposable/glass/stainless steel conjugation reactors, 1 conjugation line (Reserve space for 4 lines)
- Conjugation: 0.5 - 5 kg/batch; Annual design capacity: 300 batches, throughput 1500 kg
ADC DP GMP Manufacturing
- 1 ADC filling line: 10 m2 and 25 m2 lyophilizers
- 2 ml, 20 ml, 50 ml and full specification of aqueous injections and lyophilization isolators
- Annual production capacity: 2 million bottles; Annual output: 50 batches
One-Stop ADC CDMO Solution
Our Sites
R&D Sites


Manufacturing Sites

Case Study
We enabled the development and launch of RC48,
the first Chinese developed and approved ADC drug.

FAQs
The quotation for work starting from the new antibody sequence after screening and sequencing consists of the following components: analytical method development and validation, antibody process development (including cell line construction, cell bank construction, and Antibody process development), toxicological batch production (including one batch of 15L scale under non-gmp conditions and one batch of 100L scale under gmp conditions as well as Antibody influencing factor experiments), and registration support studies.
Members of Chongqing ChemExpress's conjugation team have been engaged in the conjugation business for over a decade, contributing to the successful market launch of multiple conjugated drugs. Meanwhile, the key personnel in the antibody production team also have more than ten years of extensive industry experience.
ChemExpress has significant advantages in both materials and registration. We have a rich inventory of payloads and intermediates, and continuously improve cost-effectiveness to ensure our products remain competitive. For the CMC of ADC small molecule part, we have supported customers in successfully completing over 50 IND projects, with 5 BLAs currently in progress. Additionally, our registration team has gained extensive experience in filing documentation in both English and Chinese, supporting dual submissions in the U.S. and China.