Drug Substance Manufacturing
Making Drug Substance More Efficient, Faster to Market
As molecules become more complex—including intermediates, highly potent APIs (HPAPIs), chiral compounds, and heterocycles—robust and scalable drug substance manufacturing processes are essential to ensure product quality, regulatory compliance, and successful commercialization in a rapidly evolving pharmaceutical landscape.
>At ChemExpress, we provide end-to-end drug substance development and manufacturing services—from route scouting and process optimization to scale-up, GMP production, and global regulatory support. Our integrated drug substance platform, including RSM, intermediate, API and HPAPI enables efficient, consistent, and cost-effective delivery from R&D to commercialization.
One-Stop Small Molecule CDMO Platform
From R&D to commercialization, we integrate CRO & CDMO capabilities across small molecules—delivering end-to-end services in RSM, intermediate, API, HPAPI and drug products.
Green Chemistry &
Enabling Technology
We specialize in complex chemistry—chiral molecules, HPAPI, heterocycles, ADC linkers, and etc.—with enabling technologies including flow chemistry, High Throughput Experimentation, biocatalysis, and Solubility-Enhancing Technologies.
Global Compliance &
Cost Efficiency
With Multiple GMP-compliant sites, we ensure regulatory excellence. Our tailored CDMO strategies help reduce costs, minimize tech transfer, and accelerate the path to market.
Our Services
At ChemExpress, we provide integrated drug substance manufacturing services covering the full development lifecycle—from early-stage route exploration and process optimization to GMP production and regulatory support. With our extensive experience,advanced technology, and robust manufacturing capacities, we support global customer’s projects across different development stages and molecular types.
Our Service Scope Includes
Route Exploration & Process Optimization
- Assessment of Synthetic Feasibility and Potential Process Alternatives
- Evaluation of Scalability, Safety, Cost, and Regulatory Considerations
- Selection of Optimal Route for Further Process Development
Process Development
- Scale-Up Process Development
- Optimization of Critical Reaction Parameters to Improve Yield and Purity
- Development of Control Strategies for Critical Process Parameters (CPP)
GMP Drug Substance Manufacturing
- Flexible GMP Manufacturing Capabilities Scales up from Grams to Tons
- Stable Supply from Clinical Stage to Commercilization
- Equipped with GMP-Grade HPAPI Production Lines and OEB-5 Isolators for Targeted Anti-tumor Drug Manufacturing.
Solid-State Chemistry
- Characterization of Polymorphs, Particle Size Distribution, Crystallinity, and etc.
- Support the Process Stability Assessment and Regulatory Filing
- Integration with Process Development and Analytical Teams
Technology Transfer
- Seamless Process Transfer Between Client and ChemExpress
- Comprehensive Documentation, Validation, and QA/QC Alignment
- Risk-based Transfer Planning with Minimized Development Delay
Analytical & Quality Control Services
- Method Development, Validation, and Transfer under ICH and GMP Standards
- Impurity Profiling, Specification Setting, and Structural Characteration
- Support for Batch Release, Stability Studies, and IND/NDA Filing
Why Partner with ChemExpress
Choosing the right CDMO partner can make all the difference in accelerating your molecule to market.
With over 900 small molecule projects successfully delivered—including 833 in preclinical and Phase I, 116 in Phase II–III, and 17 at the commercial stage—we are trusted by pharmaceutical innovators worldwide.
Looking for a reliable partner to manufacture your drug substance with speed and confidence?
Let ChemExpress be your CDMO of choice in advanced drug substance manufacturing.
Get in touch with our team today to start your drug substance journey.
Our Sites
R&D Sites

Manufacturing Sites

Case Study
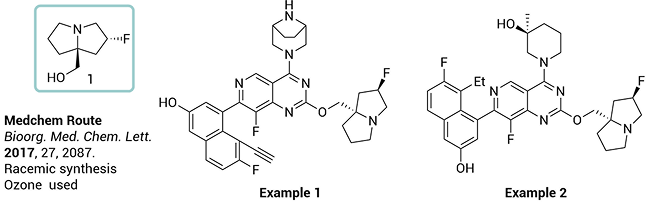
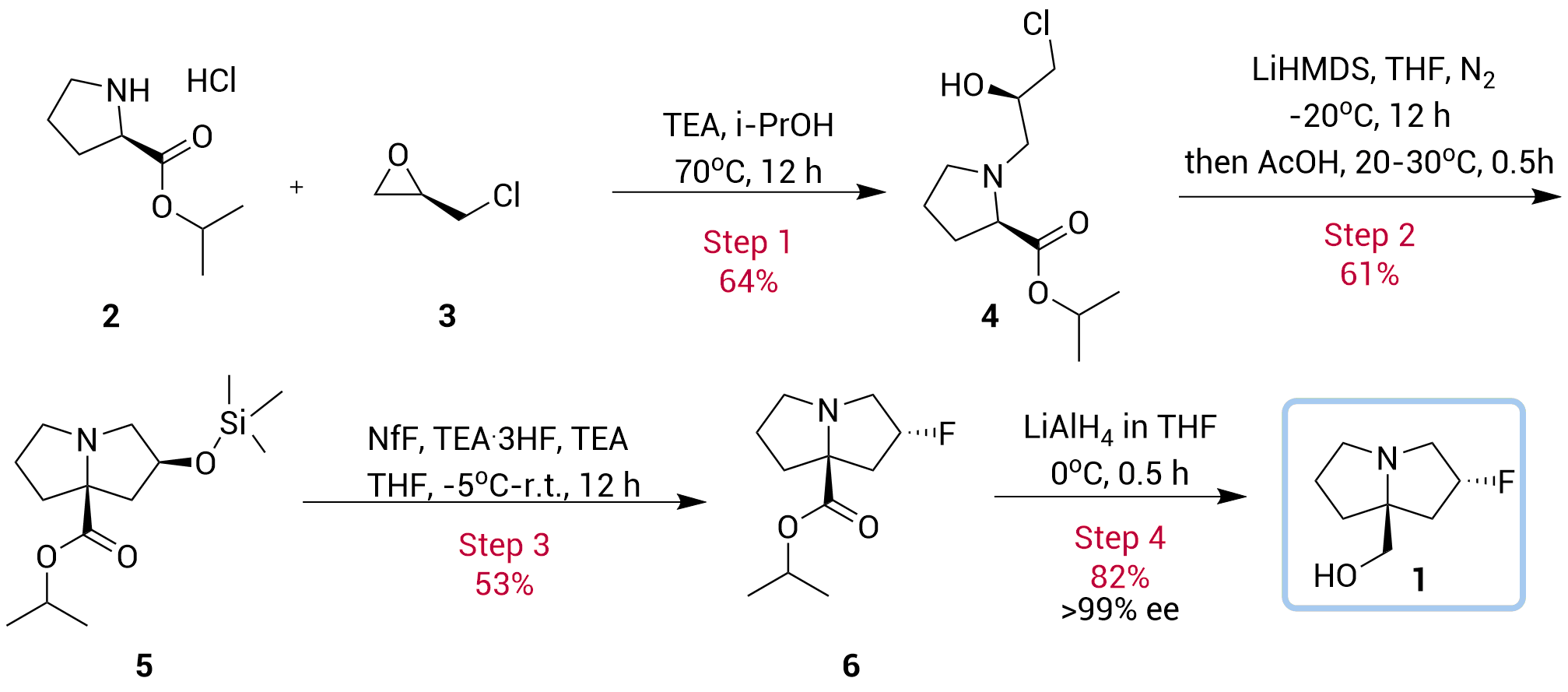
• Cost effective chiral building block
• 4-Step diastereoselective synthesis · High ee value
• The TMS ether was isolated and directly used for the fluorination
KRAS G12D is one of the most prevalent and challenging mutations to target in cancer. Therapeutics designed against this mutation typically feature a critical chiral scaffold, ((2R,7aS)-2-fluorotetrahydro-1H-pyrrolizin-7a(5H)-yl)methanol. However, existing synthetic routes to this scaffold are lengthy, suffer from low yields, and rely on hazardous reactions and costly chiral separation techniques, thereby limiting large-scale production.
To address these bottlenecks, ChemExpress has developed an efficient and scalable new process, which has been granted a PCT international patent (WO2024092420A1). Starting from inexpensive proline, the route leverages a key enantioselective epoxide ring-opening followed by an intramolecular alkylation to construct the bridged-ring structure with high selectivity. In just four steps, the target product is obtained with ee > 99%, while avoiding hazardous operations and chromatographic purification. This innovation significantly enhances production safety and cost-effectiveness, offering a reliable pathway for the industrial manufacture of such structurally challenging chiral molecules.
FAQs
These concepts form the complete small molecule drug production chain:
RSM: A fundamental chemical substance used in the synthesis of an API under GMP requirements. These components are critical in defining the structure and quality of the final API
Intermediates:Chemical substances produced during multi-step synthesis from RSMs to final API. Intermediates typically lack pharmacological activity but their quality directly impacts final API quality.
API vs Drug Substance:These terms essentially refer to the same concept—the final chemical substance with pharmacological activity. API is the traditional term more commonly used in industry, while Drug Substance is preferred by regulatory agencies like FDA and ICH in official documents. CTD submissions typically use Drug Substance, while commercial communications more often use API.
Complete workflow: RSM → Intermediate 1 → Intermediate 2 → ... → API/Drug Substance → Drug Product (formulation)
We offer end-to-end services from route scouting, process optimization, and scale-up to GMP production and regulatory support, covering RSMs, intermediates, APIs, and HPAPIs to help clients transition efficiently from R&D to commercialization.
We provide flexible GMP manufacturing capacity from milligrams to tons, supporting both early clinical supply and large-scale commercial production. Our GMP lines accommodate Phases I–III and commercial supply, equipped with OEB-5 isolators for HPAPIs.





