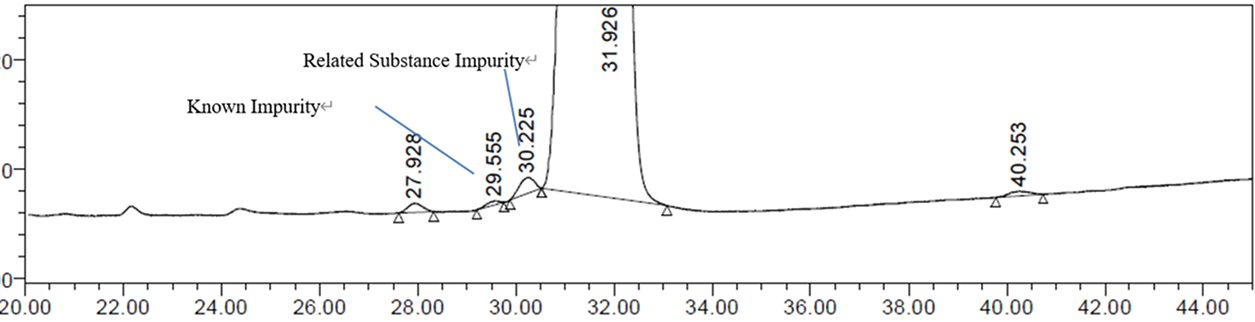
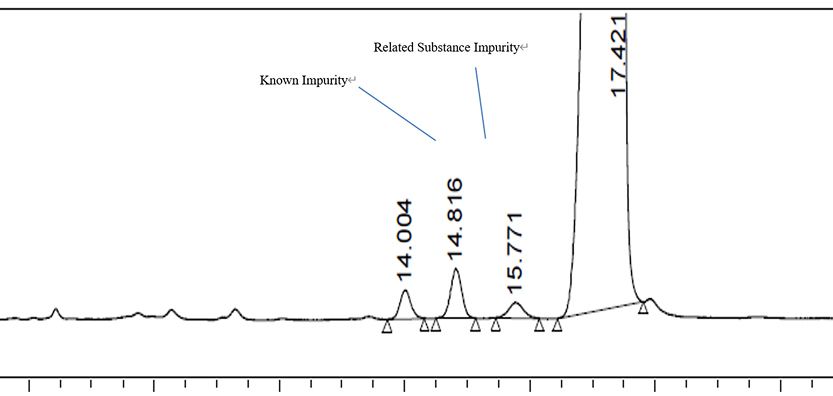
Solution for Charge Heterogeneity Analysis of ADCs
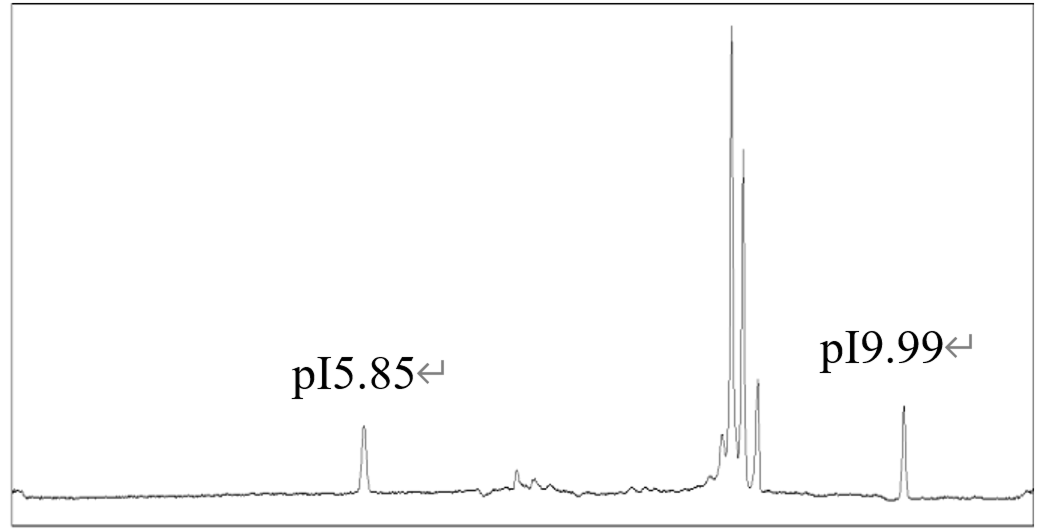
ChemExpress has developed a dedicated solution for ADC charge heterogeneity analysis based on imaged capillary isoelectric focusing (icIEF). Leveraging the principle of isoelectric focusing, icIEF enables high-resolution separation of charge variants. The method’s reliability and separation performance were further validated by the incorporation of pI 5.85 and pI 9.99 internal markers.
In this case study:
· icIEF clearly distinguished the main peak from charge variants;
· A comprehensive charge heterogeneity profile was obtained;
· Internal marker verification ensured reproducibility and accuracy.