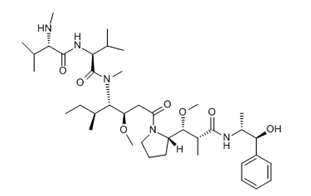
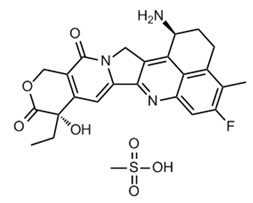
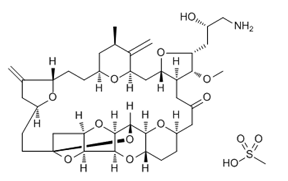
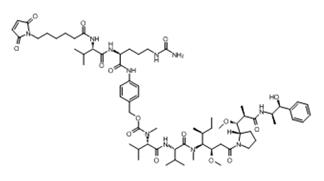
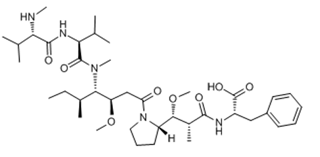
ADC Payload Linker
With over 15 years of deep expertise in the ADC Payload Linker field, ChemExpress has built a solid foundation in R&D, and has accumulated extensive experience on over 80 payloads, over 400 linkers, and over 1,000 linker syntheses experience, offering a one-stop service covering the entire ADC Payload Linker development process.

By partnering with us,
You will gain

End-to-End Solutions:
Complete control over the entire process, from Payload-Linker Syntheses to Conjugation

Efficient Process Development:
Cutting-edge technologies to accelerate the development timeline, simplify complexities, and reduce risks

Accelerating the path to market:
Leverage our extensive experience and efficient services to significantly shorten the time from R&D to commercialization
Choose us to make the development and commercialization of cutting-edge ADC drugs more efficient and reliable.

One-Stop ADC
CDMO Service
ChemExpress offers integrated services spanning from Payload-Linker to ADC DS&DP, enabling efficient scale-up and seamless technology transfer, effectively reducing management and transition costs.

Extensive Project Experience
& Comprehensive Inventory
Over 160 ADC projects have been delivered, including over 60 CMC projects, 5 BLA projects, and 1 commercial project. 15 ADC payloads and related intermediates are registered with the FDA as DMFs.

Expert Scientific
Team
Our team of over 500 scientists provides comprehensive support from R&D to commercialization, ensuring high-quality, scalable solutions for your ADC pipeline.
Our Services
ChemExpress offers comprehensive technical services covering the entire lifecycle of ADC payloads & linkers, from R&D to commercialization, accelerating the path to market for your innovative drugs.
Our Service Scope Includes
Molecular Synthesis Route Development
- Experience-Based Optimization
- Rapid Syntheses Route Development & Milligram-Scale Sample Delivery
Process Development & Optimization
- Route Screening & Optimization
- Stage-Specific Process Strategy:
· IND Stage: Focus on GLP toxicology and GMP clinical batch production, accelerating clinical submission
· BLA Stage: Optimize production conditions for cost-efficiency and assess quality risks to ensure robust processes
- Seamless Transition: From milligram-scale exploration to kilogram-scale GMP production
- Process Safety Assessment
- Tech Transfer & Scale-Up
Analytical Development & Quality Research
- Comprehensive Analytical Support: Method development, optimization, transfer, and validation, including in-process control (IPC) and product release testing
- Impurity Studies: Identification and control of process-related impurities, including genotoxic and chiral impurities, in compliance with ICH M7
GLP/GMP Production & Compliance
- Professional Production System
- Stability Studies
- EHS Compliance
- Process Validation Support
Regulatory Registration Strategy & Support
- Expert Regulatory Guidance: Providing regulatory interpretation and submission strategy consultations
- Registration Documentation: High-quality CMC documentation drafting support
- 60+ CMC Projects, 5 BLA Projects and 1 Commercial Project
Professional Project Management Support
- Dedicated Project Manager
- Seamless Cross-Department Coordination
- Project Execution & Timely Updates
Why Partner with ChemExpress
Comprehensive End-to-End Solutions
Over 80 payloads, over 400 linkers, and over 1,000 linker syntheses experience, enabling the management of ADC Payload-Linker CMC challenges from R&D to commercialization, and providing a seamless, one-stop service to accelerate market entry
Minimized Risk
Expertise in impurity research, EHS compliance, process safety assessments, and robust process development to minimize technical, regulatory, and safety risks
Extensive Projects Exeperience
15 ADC payloads and related intermediates are registered with the FDA as DMFs, supported over 60 CMC Projects, 5 BLA projects and 1 commercial project
Uncompromising Compliance & Quality Assurance
Strict compliance to GLP/GMP, backed by a powerful quality management system and regulatory support, passing 1 US FDA inspections with zero 483 observations and 1 EU third party QP GMP audit
Professional Team Support
Access to a team of over 500 experts in synthetic chemistry, process development, analytical sciences, manufacturing operations, and regulatory affairs, ensuring project success
Our Sites
R&D Sites


Case Study
Eribulin Mesylate
• Breakthrough optimization of key steps in the synthetic process
• Completed 100-gram GMP process verification and stability test of API and advanced intermediates
• Characteristic impurity limit < 0.1%
• Stable supply of 100 gram (GMP & non-GMP) for API and advanced intermediates
• DMF code: MF033836, MF037054, the project has been approved by ASMF and USDMF
Vc-MMAE
• Chiral control from key intermediate to final product
• Quality: ≥ 99% purity, ≥ 99% ee, ≥99% de, each impurity < 0.1%
• Stable supply delivery of intermediates and Vc-MMAE (various specs) as well as mc-vc-PAB-MMAE in kg qualities
• DMF code: MF035548,MF035549,MF035550,MF042645,MF042644,MF040956,MF042170
FAQs
Based on our typical project experience, we provide the following estimated timeline for your reference and planning:
· Process Optimization & Demo Production: 3 - 4 months.
Includes optimizing the synthesis route, demo-scale production verification, and toxicology batch production.· Process Transfer & Technical Package Preparation: 1 month.
Includes synthesis method transfer and process safety assessment.· GMP Production Preparation & Execution: 1 - 2 months.
Includes production scheduling, material procurement, GMP production, and release testing.· Registration Filing Documentation Preparation: 1 - 2 months post-GMP production.
The registration team will intervene early to collect data and draft the quality sectionWe not only possess the full set of advanced equipment and specialized technologies needed to address complex chemical structures, instability, and unique physicochemical properties (e.g., precision chromatography, lyophilization purification, narrow pH control), but more importantly, we have accumulated extensive project experience. This allows us to effectively integrate and apply these technologies, solving real development challenges, ensuring efficient and stable production of Payload-Linkers that meet stringent quality standards, and providing a solid CMC foundation for the successful development of ADC drugs.
Cost challenges are a common pain point in ADC drug development. Understanding the cost structure is the first step. The main driving factors for the high cost of ADC Payload-Linker are summarized as follows:
· Compound Complexity: Complex structures (multiple chiral centers, large molecular weight 1000-5000 Da), special physicochemical properties (both hydrophilic and lipophilic, chemically unstable, difficult to crystallize), resulting in long synthesis routes, challenging purification, and time-consuming process development.
· Small-Scale Demand:: As a key component of ADCs, the quantity required is extremely low compared to antibodies (molecular weight 150k-200k+), typically in milligram to gram per batch quantities. This makes it impossible to dilute fixed costs through large-scale production, which is fundamentally different from traditional APIs (kilogram to ton scale).
· Highly Potent API Control: The extremely high cytotoxicity of these substances demands strict containment production, specialized facilities, personnel protection, waste management, and environmental monitoring. These GMP-level engineering and management inputs are costly, limit production speed, and increase batch turnaround times.