Small Molecule CDMO
-
Small molecules remain a dominant force in the pharmaceutical industry due to their high bioavailability, well-established production processes, and regulatory clarity. In this landscape, small molecule CDMO plays a critical role in accelerating innovation, managing complexity, and ensuring scalable production.
As a reliable small molecule CDMO partner, ChemExpress supports pharmaceutical and biotech clients’ projects across the full lifecycle of drug development—from early discovery and process development to commercialization. With a strong commitment to innovation and regulatory compliance, we deliver high-quality solutions including RSM, intermediate, API, HPAPI, and drug products. Our integrated platform helps accelerate the journey of small molecule from R&D to commercialization.
One-Stop Small Molecule CDMO Platform
From R&D to commercialization, we integrate CRO & CDMO capabilities across small molecules—delivering end-to-end services in RSM, intermediate, API, HPAPI and drug products.
Green Chemistry &
Enabling Technology
We specialize in complex chemistry—chiral molecules, HPAPI, heterocycles, ADC linkers, and etc.— with enabling technologies including flow chemistry, High Throughput Experimentation, biocatalysis, and Solubility-Enhancing Technologies.
Global Compliance &
Cost Efficiency
With Multiple GMP-compliant sites, we ensure regulatory excellence. Our tailored CDMO strategies help reduce costs, minimize tech transfer, and accelerate the path to market.
Our Services
ChemExpress combines deep chemistry expertise with global regulatory compliance experience, supporting the small molecule CDMO landscape to accelerate development and minimize risk—empowering our clients to seize market opportunities faster.
Our Service Scope Includes
Drug Discovery Services
- Skilled in Complex Synthesis Routes
- Full-Time Equivalent (FTE) and Fee for Service (FFS) Services
- Cost-Effectiveness and Efficient Delivery
- IP Protection
Process Development
- Route Scouting and Optimization
- Scalable and Cost-Effective Processes
- Seamless Transition to GMP Manufacturing
- End-to-End Tech Transfer and Scale-Up Support
Drug Substance Manufacturing
- Therapy Area: Anti-tumor, antiviral, diabetic, cardiovascular ,
gastrointestinal tract, cerebrovascular diseases and etc. - cGMP-Compliant Facilities
- Outstanding Synthesis Capability
Highly Potent API Manufacturing
- OEB-5 Compliant Workshop
- Rich Experience in HPAPI Handling
Drug Product Manufacturing
- Oral Solid: tablets, hard capsules, granules, powders; Semi-solid:
creams, ointments and gels - cGMP Manufacturing
- Solubility-Enhancing Technologies (Hot-Melt Extrusion, Spray Dryer)
- 20 Years of Rich Experience
- Served >200 New Drug Formulation Projects
Regulatory CMC Support
- IND/NDA Dossier Preparation
- US-China Dual IND Filing Support
- eCTD-Compliant Document Preparation
- Communication Support with Regulatory Authorities
Analytical Development & Quality Control
- Comprehensive Coverage of Method Development and Validation
- Stability Studies and ICH-Compliant Testing
- Release Testing for RSM, Intermediate, API, and Drug Product
- Fully Equipped with Analytical Instruments
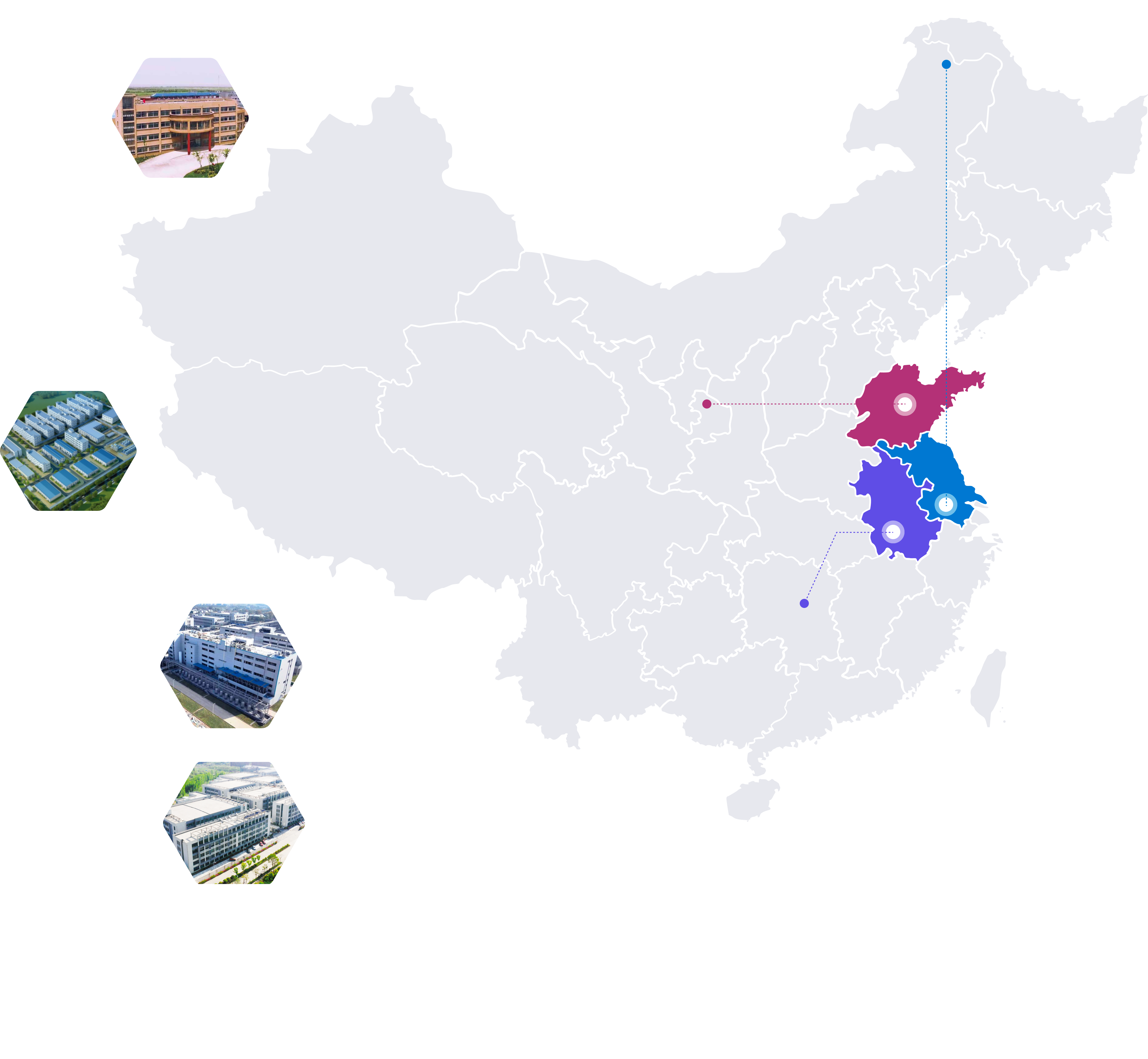
Our Sites
R&D Sites





Manufacturing Sites
Drug Product Process Development and Manufacturing
•Total area: ~139,930 ft²
•Oral solid dosages: 4 commercial-scale workshops, ~1 billion units per year
•Semi-solid topical dosages: 3 commercial-scale workshops, ~50 million tubes per year
•Solid dispersion: 1 large scale topical preparation line
•Formulation development and manufacturing: tablets, hard capsules,
granules, powders, creams, ointments and gels, including anti-tumor drugs
Specialty chemicals, Intermediates Manufacturing
•Reactors: ~50 reactors range from 500L - 5,000L
•Scale up to hundreds of kgs per batch
•Total reactor volume: ~115,000L
Site1: Anhui Ma‘anshan R&D Center
ADC Payload-Linkers & HPAPIs Process Development and Manufacturing
•5 GMP HPAPI production lines (OEB5, OEL 0.01μg/m³) with a total volume over 4,000L
•2 API GMP kilogram scale production line
•6 Class D clean room in total, scale up to kilograms (commercial scale)
•Multiple negative pressure isolators, prep-HPLC and Lyophilizers
Site2: Anhui Ma'anshan c-GMP Manufacturing Site
RSMs, Advanced Intermediates, APIs and Peptides Process Development and Manufacturing
•Number of reactors: ~170, range from 100L-8,000L
•Total reactor volume:~483,000L
•Reactions: Hydrogenation reaction, Grignard reaction, oxidation reaction,
photoreaction, high temperature
reaction(240°C) and deep cryogenic reaction (-80°C)
•Multiple negative pressure isolators, prep-HPLC and Lyophilizers
Case Study
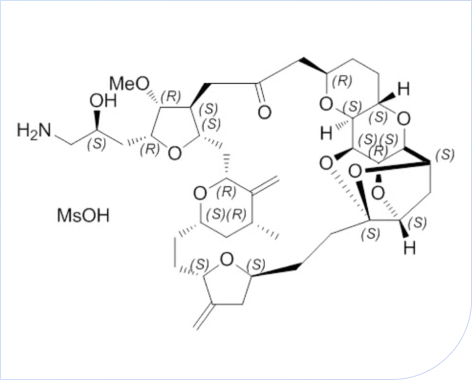
Breakthrough Optimization in the Synthesis of Eribulin:
19 chrial centers, 62 steps synthesis process
• Completed 100-gram GMP process verification and stability test of API and advanced intermediates
• Characteristic impurity limit < 0.1%
• Stable supply of 100 gram (GMP & non-GMP) for API and advanced intermediates
• DMF code: MF033836, MF037054, and the project has been approved by ASMF and USDMF
FAQs
An API is the chemically active substance in a medication that produces the intended therapeutic effect. Drug products (formulations) combine APIs with excipients (such as starch, lactose) in specific ratios to create the final dosage forms for patient use, including tablets, capsules, and injections. Simply put, APIs are the "active components" while drug products are the "finished medicines."
We possess deep expertise in complex small molecule synthesis, specializing in challenging chemical structures including chiral molecules, highly potent APIs (HPAPIs), heterocyclic compounds, and ADC payloads. Our advanced technology platforms encompass continuous flow chemistry, high-throughput experimentation (HTE), biocatalysis, and bioavailability enhancement technologies. We maintain comprehensive analytical capabilities ensuring end-to-end quality control from starting materials to final products.
We have established a comprehensive cGMP quality management system with manufacturing facilities compliant with international regulatory standards including FDA, EMA, and NMPA requirements. We rigorously follow ICH guidelines and have implemented complete quality risk management and deviation handling procedures. Our professional stability study capabilities support clients throughout the entire regulatory journey from IND to NDA, facilitating successful regulatory approvals across major global markets.