ADC Drug Substance Process Development
The rising wave of ADC innovation is reshaping the development of targeted therapies, with the global ADC market projected to reach USD 68.5 billion by 2030, growing at a CAGR of over 30%*. This rapid expansion is driving demand for robust, scalable, and cost-effective ADC process development.
But behind every successful ADC lies a complex ADC drug substance development process involves critical stages such as Payload-Linker Synthesis, Antibody Production, and ADC Conjugation. These elements form the foundation of high-quality, efficient ADC process development.
At ChemExpress, we integrate advanced chemical synthesis, biologics expertise, and innovative conjugation technologies on our Quality by Design (QbD) driven platform, offering faster, safer, and more cost-effective ADC drug substance process development solutions in today’s competitive landscape.
*Source: Frost & Sullivan 2025 China Pharmaceutical CDMO Industry Insight Blue Book

One-Stop ADC
CDMO Service
ChemExpress offers integrated services spanning from Payload-Linker to ADC DS&DP, enabling efficient scale-up and seamless technology transfer, effectively reducing management and transition costs.

Extensive Project Experience
& Comprehensive Inventory
Over 170 ADC projects have been delivered, including over 70 CMC projects, 5 BLA projects, and 1 commercial project. 16 ADC payloads and related intermediates are registered with the FDA as DMFs.

Expert Scientific
Team
Our team of over 600 scientists provides comprehensive support from R&D to commercialization, ensuring high-quality, scalable solutions for your ADC pipeline.
Our Services
Partner with us for cutting-edge, efficient ADC drug substance process development, from payload-linker synthesis to conjugation. We provide customized ADC process development solutions that simplify complex challenges, minimize risks, and accelerate the path to market — so you can bring your innovations to life faster.
Our Service Scope Includes
ADC Payload Linker Synthesis
- Process Parameter Optimization
- Stability Research on Unstable Intermediates
- Purification Method Research
- Impurity Control Strategy Research
- Process Characterization & Validation
Antibody Process Development
- Fast Process Development
- Optimized Expression & Purification
- Antibody Freeze-Thaw Assay
- Intermediate Stability
- Antibody Process Characterization
- Comprehensive Scale-up Support
Conjugation Process Development
- Various Conjugation Technologies, such as Cysteine-based Conjugations (-SH), Lysine-based Conjugations (-NH2), N-Glycan Conjugation and Enzyme Conjugations
- Dual Payload Conjugation
- Characterization of the Drug Substance Process
- Stability of Coupling Intermediates
- DAR (2.0/4.0/8.0±0.3) Precise Control
- Drug Substance Freeze-Thaw Assay
- Free Drug Control
- Scalable Conjugation Process
- Drug Substance Process Characterization & Validation
- Smooth Technology Transfer to GMP
Why Partner with ChemExpress
One-stop Service
Seamless solutions from payload-linker, antibody to ADC drug substance, through modular process development,13 months from DNA to IND, optimizing efficiency and scalability, ensuring your project meets milestone
Flexible Execution & Cost Effective
Flexible execution with rapid turnaround, supported by seamless integrated service, effectively reducing management and transition costs
Precise Control
Customized DAR distribution, with precise control (2.0/4.0/8.0±0.3) and >98% monomer purity (SEC-HPLC verified), ensuring high-quality and reproducible results
Versatile Conjugation Solutions
Comprehensive conjugation solutions for Cysteine-based conjugations (-SH), Lysine-based conjugations (-NH2), N-Glycan Conjugation and Enzyme conjugations, supporting both non-site-specific and site-specific approaches, offering tailored solutions, including SEC-HPLC, DAR and quality control of free payload, etc
Different Scales of Conjugation Reactors
Equipped with 50L、100L、200L and 500L process development conjugation reactors to meet diverse conjugation scale requirements, with cross-scale performance stability validated for seamless process transfer
Green Chemistry
EcoVadis Silver certification, Green chemistry initiatives for improved efficiency
Our Sites
R&D Sites


Manufacturing Sites

Case Study
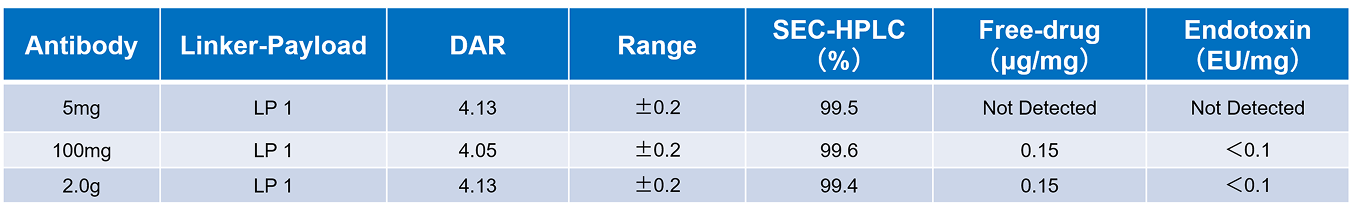
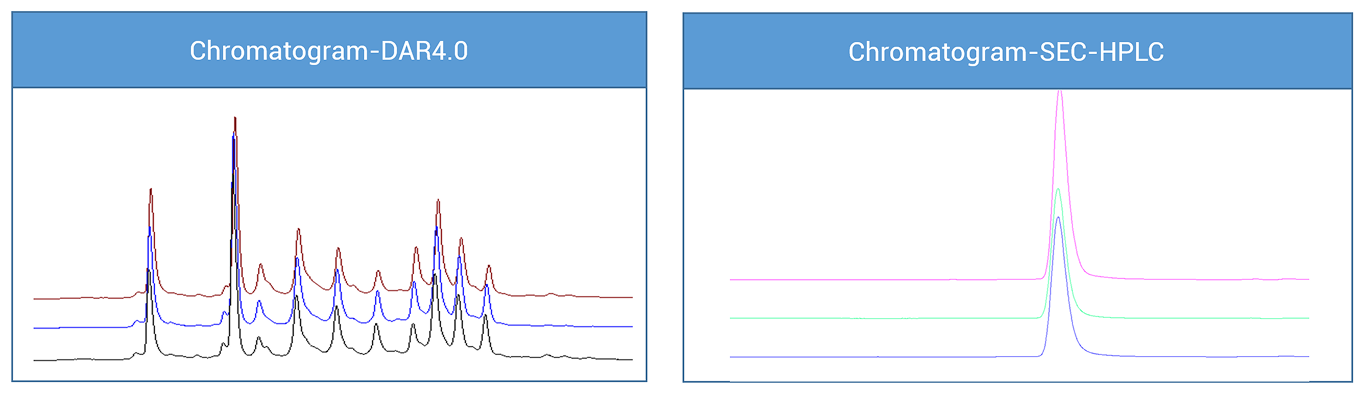
During the process development of the bispecific antibody (DAR4) conjugation, Our sccientist found that as the reaction scale increased from 20 mg, 300 mg, and gradually up to 3.0 g, the stability of the conjugate product demonstrated good controllability. However, initial analyses still indicated risks of small molecule residuals and variation in endotoxin levels. To further improve product quality and ensure stability under various process conditions, the team implemented a rapid optimization strategy. Particularly in the control of small molecule residuals and endotoxin levels, through optimizing conjugation reaction conditions and purification processes, the team successfully achieved the following results:
· Post-conjugation SEC-HPLC purity: greater than 99%
· Small molecule residuals: below 0.11 μg/mg
· Endotoxin impurity limitt: below 0.1 EU/mg
FAQs
This is a critical question regarding the production process of ADC. In simple terms, ADC Process Development is an umbrella term that encompasses the complete development process from antibody-linker-payload the final drug product formulation. It can be clearly divided into two core stages:
1. ADC Drug Substance Process Development
· Focus: This stage concentrates on the production of the API of the ADC, i.e., the "naked drug" before it undergoes any final formulation processing.
· Core Content: The primary focus is on the development and optimization of the conjugation reaction, where the antibody is attached to the drug payload.
2. ADC Formulation Development
· Focus: This stage focuses on converting the ADC drug substance produced in the previous step into a final drug product suitable for clinical use.
· Core Content: The primary focus is on ensuring the stability, safety, and suitability of the final dosage form.
Therefore, ADC Process Development defines the entire technical pathway from raw materials to the final drug product, outlining both the manufacturing process for the drug substance and the formulation of the final product.
We have recently been working on the preparation of bispecific antibody samples for the PCC stage, with experience in two bispecific antibody ADC projects so far. Most of the R&D and production team members also have experience with bispecific antibodies. The technical validation of dual-payload and the preparation of early-stage samples have been completed.
The thioether bond formed between maleimide and thiol groups may undergo retro-Michael addition in the presence of exogenous thiol-containing molecules, such as cysteine on serum albumin and glutathione (GSH). This instability can compromise the circulatory stability of the drug, increase off-target toxicity, and reduce the efficacy of antibody-drug conjugates (ADCs). Solutions: Hydrolytic ring-opening or modifying the payload (PL) by incorporating PEG and other modifications.






