Key Technology Platforms
ChemExpress has established the following technology platforms to support our API and formulation process development and manufacturing projects from laboratory scale to commercial scale.
-

 High-Throughput Experimentation (HTE)HTE is a well-proven tool that allows for the setup, execution, and analysis of many carefully chosen reactions in parallel, with less material and effort per experiment than traditional experimentation. ChemExpress has established HTE platform to keep up with the increasing demand of pharmaceutical timelines in drug discovery and development.Read More
High-Throughput Experimentation (HTE)HTE is a well-proven tool that allows for the setup, execution, and analysis of many carefully chosen reactions in parallel, with less material and effort per experiment than traditional experimentation. ChemExpress has established HTE platform to keep up with the increasing demand of pharmaceutical timelines in drug discovery and development.Read More -

 BiocatalysisEnzymes are increasingly used in large-scale drug manufacturing due to their high efficiency, specificity, and cost-effectiveness. ChemExpress comprehensive biocatalysis platform offers a one-stop solution for all of your biocatalysis challenges; this includes enzyme screening, fermentation, process development, and commercial production. We have a vast enzyme library with over 800+ enzymes available for rapid screening. Our production scale ranges from hundreds of grams to hundreds of kilograms.Read More
BiocatalysisEnzymes are increasingly used in large-scale drug manufacturing due to their high efficiency, specificity, and cost-effectiveness. ChemExpress comprehensive biocatalysis platform offers a one-stop solution for all of your biocatalysis challenges; this includes enzyme screening, fermentation, process development, and commercial production. We have a vast enzyme library with over 800+ enzymes available for rapid screening. Our production scale ranges from hundreds of grams to hundreds of kilograms.Read More -

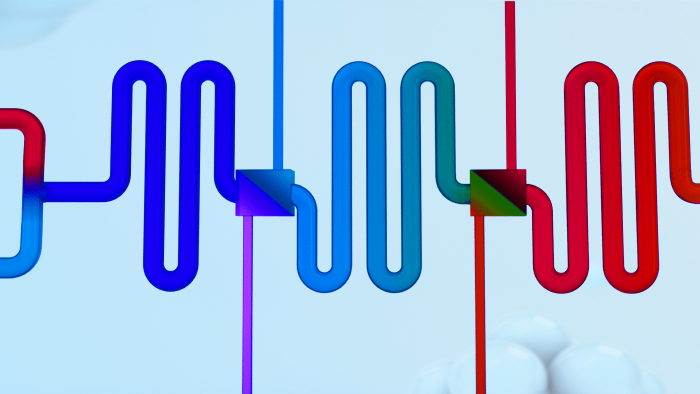 Flow ChemistryFlow chemistry(Continuous flow chemistry) refers to the continuous flow state by adjusting the key parameters of the reaction (temperature, pressure, pH, wavelength, residence time, etc.), continuous accomplishment of reaction transformation, extraction, crystallization and other processes.
Flow ChemistryFlow chemistry(Continuous flow chemistry) refers to the continuous flow state by adjusting the key parameters of the reaction (temperature, pressure, pH, wavelength, residence time, etc.), continuous accomplishment of reaction transformation, extraction, crystallization and other processes.
Common types of flow chemistry: plug-flow reactor(PFR), continuous stirred tank (CSTR), MicroPacked-bed, Micro-reactor, etc.Read More -

 Solid State Chemistry ResearchOur solid-state chemistry research technology platform has served many pharmaceutical companies around the world. It has successfully promoted the process from free form polymorph screening to the scale-up production of commercial ton-scale.Read More
Solid State Chemistry ResearchOur solid-state chemistry research technology platform has served many pharmaceutical companies around the world. It has successfully promoted the process from free form polymorph screening to the scale-up production of commercial ton-scale.Read More -

 Preparative Chromatography ServiceChemExpress offers one-stop preparative chromatographic stand-alone services for separation, purification and production of APIs and advanced intermediates under GMP. We are equipped with prep-HPLC and prep-SFC production systems that can support milligram to kilogram scale production.Read More
Preparative Chromatography ServiceChemExpress offers one-stop preparative chromatographic stand-alone services for separation, purification and production of APIs and advanced intermediates under GMP. We are equipped with prep-HPLC and prep-SFC production systems that can support milligram to kilogram scale production.Read More -

 PhotochemistryTraditional batch type photoreactor is difficult to achieve large-scale production through photocatalytic reaction. ChemExpress photocatalytic continuous flow platform can provide process route development and optimization for photochemical reaction projects, achieving milligram to gram scale process development; also it can provide large-scale production of photochemical reaction products, achieving production from kilogram to hundred kilogram scale.Read More
PhotochemistryTraditional batch type photoreactor is difficult to achieve large-scale production through photocatalytic reaction. ChemExpress photocatalytic continuous flow platform can provide process route development and optimization for photochemical reaction projects, achieving milligram to gram scale process development; also it can provide large-scale production of photochemical reaction products, achieving production from kilogram to hundred kilogram scale.Read More -

 Spray Dried DispersionFor the active pharmaceutical ingredients (APIs) that fall into BCS (biopharmaceutical classification system) class II and IV spaces, advanced formulation approaches are required to enhance the aqueous solubility and bioavailability. Amorphous solid dispersions (ASDs) via spray drying is one of the enabling technologies.
Spray Dried DispersionFor the active pharmaceutical ingredients (APIs) that fall into BCS (biopharmaceutical classification system) class II and IV spaces, advanced formulation approaches are required to enhance the aqueous solubility and bioavailability. Amorphous solid dispersions (ASDs) via spray drying is one of the enabling technologies.
We have mg to gram scale spray drying equipment to enable formulation scientists to include spray-dried dispersions (SDDs) early in compound screening stage. We are equipped with GMP pilot and production scale (kgs to tons) equipment supporting drug development, clinical trial sample preparation and commercial production.Read More -

 Hot Melt ExtrusionThe mechanism of HME is to disperse APIs in the polymer matrix at the molecular level to form amorphous solid dispersions. Amorphous solid dispersions can improve bioavailability in more than 80% of cases. The advantage of HME technology is the capability of continuous manufacturing with solvent-less processing and friendly downstream processing of extrudates into final dosage forms (tablets, capsules, controlled release forms etc.). HME has increased use in the pharmaceutical industry.Read More
Hot Melt ExtrusionThe mechanism of HME is to disperse APIs in the polymer matrix at the molecular level to form amorphous solid dispersions. Amorphous solid dispersions can improve bioavailability in more than 80% of cases. The advantage of HME technology is the capability of continuous manufacturing with solvent-less processing and friendly downstream processing of extrudates into final dosage forms (tablets, capsules, controlled release forms etc.). HME has increased use in the pharmaceutical industry.Read More



